Protein Stability Study Services
Understand the shelf life of your protein or antibody with stability testing services from antibodies-online and our partner Rockland Immunochemicals, Inc. (Rockland).
We support a variety of reagent stability testing needs with real time stability studies, accelerated stability studies, and open stability studies, all performed to the highest standards so you can quickly determine the projected lifetime and expiration date of your product. We recommend conducting stability testing for all critical reagents used in your kits and in vitro diagnostics including antibodies, enzymes, and other biologics.
Real Time Stability Studies
Our real time stability testing services are used to confirm or establish long-term stability of your product. We evaluate your protein or antibody’s performance based on physical characteristics and activity over the projected lifetime to determine the expiration date. We conduct real time studies based on your defined requirements with time points for 6 months, 1 year, or as many as 2–5 years.
Open Stability Studies
The open or "in use" stability testing is designed to mimic the effects of normal use, such as freeze/thaw cycles, reconstitution, and/or aliquot size. This study will help you understand and give guidance to end users of your product. Our open stability studies are measured using industry standard reproducibility measurements including precision and %CV. All stability studies are performed with our partner Rockland and can include customized reports or reports using your defined reporting template.
Start Your Project
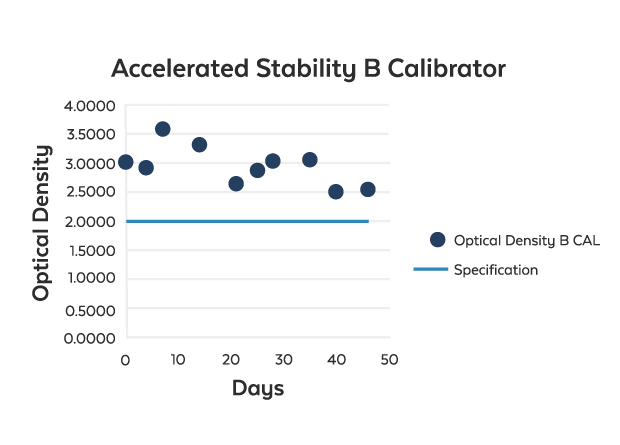
Accelerated Stability Studies
Rockland's accelerated stability testing can help you bring a new product to market as quickly as possible. With accelerated stability testing, we subject the product to an elevated level of stress such as through controlled changes in temperature or humidity. The testing period is determined by the time frame expected or required for the expiration of the product (i.e. 1 year, 2 years, etc).
Accelerated stability testing data is recognized by international regulatory bodies as an acceptable means to immediately generate required data, but is only accepted when those tests can be repeated on "real time" samples. Thus, we recommend running a real-time stability test in tandem with the accelerated stability test.

Our Service Partner: Rockland
Our Service Partner: Rockland
Benefit from expertise over two generations of scientific advances. Rockland enabling biomolecule detection, characterization and analysis since 1962. 60,000 square feet manufacturing facility produce high-quality antibodies, reagents and custom services for your next project
Benefit from expertise over two generations of scientific advances. Rockland enabling biomolecule detection, characterization and analysis since 1962. 60,000 square feet manufacturing facility produce high-quality antibodies, reagents and custom services for your next project




